- Know What You Need, Get What You NeedTim Loughran CleanRooms BASICS magazine December 2002 Ignore the preconceived criteria and determine what is truly required for your cleanroom project
Understanding the Cleanliness and Finish Quality Requirements for Food Processing Facilities
The demand for ever increasing quality in food manufacturing has set forth with requirements that are demanding more stringent sanitary guidelines for the facilities. Current food good manufacturing practices (GMPs) are published in Title 21 of the Code of Federal Regulations, Part 110 (21 CFR 110). GMPs describe the methods, equipment, facilities, and controls for producing processed foods. As the minimum sanitary and processing requirements for producing safe and wholesome food, these GMPs are an important part of regulatory control over the safety of the nation’s food supply. GMPs also serve as one basis for FDA inspections for the manufacturing facilities, and should be integrated and considered in the design and construction of factory.
In general a facility should meet the following criteria, to meet the requirements of 21 CFR Part 110:
- Be constructed in such a manner that floors, walls, and ceilings may be adequately cleaned, and kept clean, and kept in good repair; that drip or condensate from fixtures, ducts and pipes does not contaminate food, food-contact surfaces, or food-packaging materials; and that aisles or working spaces are provided between equipment and walls and are adequately unobstructed and of adequate width to permit employees to perform their duties and to protect against contaminating food or food-contact surfaces with clothing or personal contact.
- Provide adequate lighting in hand-washing areas, dressing and locker rooms, and toilet rooms in all areas where food is examined, processed, or stored and where equipment or utensils are cleaned; and provide safety-type light bulbs, fixtures, skylights, or other glass suspended over exposed food in any step of preparation or otherwise protect against food contamination in case of glass breakage.
- Provide adequate ventilation or control equipment to minimize odors and vapors (including steam and noxious fumes) in areas where they may contaminate food; and locate and operate fans and other air-blowing equipment in a manner that minimizes the potential for contaminating food, food-packaging materials, and food-contact surfaces.
- Cleaning compounds and sanitizing agents used in cleaning and sanitizing procedures shall be free from undesirable microorganisms and shall be safe and adequate under the conditions of use.
- Provide adequate floor drainage in all areas where floors are subject to flooding-type cleaning or where normal operations release or discharge water or other liquid waste on the floor.
- Providing self-closing doors that do not open into areas where food is exposed to airborne contamination, except where alternate means have been taken to protect against such contamination (such as double doors or positive air-flow systems).
- Hand-washing facilities shall be adequate and convenient and be furnished with running water at a suitable temperature. Compliance with this requirement may be accomplished by providing hand-washing and, where appropriate, hand-sanitizing facilities at each location in the plant where good sanitary practices require employees to wash and/or sanitize their hands. In addition, provide sanitary towel service or suitable drying devices. Devices or fixtures, such as water control valves, so designed and constructed to protect against recontamination of clean, sanitized hands.
- Provide refuse receptacles that are constructed and maintained in a manner that protects against contamination of food.
DESIGN GUIDELINES
The objectives of designing and constructing a sanitary food handling facility are to minimize harborages and eliminate the entrance of pests and other sources of contamination. The role of rodents, insects, birds, and other pests in spreading foodborne pathogens has been well documented. It is imperative that an adequate pest control management program is in place for food processing and handling facilities. The primary objective of sanitary design in building construction is to design and construct a building that is cleanable. Other major considerations are to minimize contamination and adequately seal food processing and handling areas from sources of contamination. Receiving areas and rooms should be enclosed as much as is practicable. Ideally, loading docks should be at least 3 feet above ground with the underside lined with a smooth, galvanized metal or similar material with a 12 inch over-hang. Properly installed rapid open/close doors or air curtains should be used to discourage entrance of insects and birds. Building materials used for exterior walls vary in their need for preventative maintenance with regard to re-caulking of joints. Concrete block walls should be sealed at the base and capped at the top. The roof should be designed and built so it can be kept clean. Openings into buildings, including doors, windows, ventilation ducts, and other openings must be appropriately sealed and protected. Openings into the roof such as exhaust fans for air handling systems, ventilation ducts, and plumbing vent pipes must be sealed, and appropriately flashed and screened. Windows are discouraged in food processing operations as they present sanitation problems due to glass breakage and overall maintenance consider¬ations. If used, windows should be designed to be flush with the inside wall and be permanently closed. Sills should be sloped away from the wall at not less than a 45 degree angle to prevent birds from nesting or dust from collecting.
The sanitary objectives for interior building design and construction are to minimize potential harborages of pests and microorganisms, maximize clean-ability; and enhance the protection of the food products from contamination. A new facility is easily built with sanitary design criteria in mind. Building designers can integrate sanitary objectives without adding a great deal of cost to a construction project. Existing facilities present a different set of challenges due to construction practices that are now considered obsolete, and cost considerations for updating these facilities. Having a facility designed and built to sanitary specifications does not guarantee a safe food product if the facility is not adequately cleaned and maintained on an appropriate schedule. A cleanable, sanitary wall is one that is hard, flat, and smooth, free of pits, cracks, checks, and crevices, impervious and non-absorbent, resistant to cleaning and sanitizing chemicals, corrosion resistant, durable, easily maintained, and wear resistant; and properly installed, sealed, and covered. The wall should be installed and maintained to assure these properties are met from the floor to the ceiling. In addi¬tion, if used openings and windows are used they should be installed and sealed to maintain the floor to ceiling properties. There are several acceptable surfaces and materials available for walls in food processing and handling areas. Fiberglass Panels are a highly acceptable wall material and are commonly used in newly constructed facilities. While most of the available panels are acceptable; gel coated, reinforced fiberglass panels are most recommended. This material, when properly installed and sealed at the seams, provides a continuous, hard, durable, and cleanable surface. However, if fiberglass board is improperly installed, improperly maintained, or becomes damaged, it can lose its desirable features. If the panels extend to the floor, they are vulnerable to damage from forklifts and related equipment. In high impact locations, a concrete curb may be recommended. To prevent creation of a ledge (which will collect dust) the top of the curb should be sloped at a 45 degree angle or greater or the curb constructed in a bull nose design.
Wood (e.g., plywood, pressed wood), due to its porosity, is not recommended and should be avoided for interior walls in food facilities. Wood cannot be adequately sealed. Walls should be caulked, sealed, grouted appropriately at joints and junctions. Such coverings and sealants are used to enhance the impervious properties, cleanability, and ease of main¬tenance. A preventative maintenance program should be in place to keep walls in good repair. Junctures between walls and ceilings, and between walls and floors should be rounded (or coved) with a radius of one inch or greater. Coving minimizes a right angle crevice, which is difficult to clean and maintain.
Ceilings in food handling facilities, are often neglected from a sanitary design and construction perspective, and should meet the same objectives mentioned for walls. In addition, they need to be included in a preventative main¬tenance program. Improperly installed ceilings, ceilings that promote condensation, or poorly maintained ceilings (e.g., flaking paint) can actually increase the potential for overhead contamination of food products. Dropped ceilings are acceptable only if properly installed. False ceilings, which create a crawl space above the ceiling for utilities and services, should be avoided. The crawl space also becomes very attractive to insects and rodents, increas¬ing the potential of product contamination. Because fiber¬glass panels can be glued in and sealed at the joints they are acceptable in dropped ceiling application; however, they are very difficult to maintain in a continuously sealed condition. Permanent dropped ceilings, which essentially create a walk-on second floor above the processing area, are more desirable. The additional floor is used to run utilities, air handling ducts, fans, and similar services. Utilities are often conveniently located in this upper floor over a dropped ceiling and away from the processing area. Pipes, conduits, and similar accoutre-ments can be installed in vertical runs through the ceiling into the upper floor; however, the junctures of these pipings need to be properly sealed and the seals maintained in good repair.
Insulation materials available do not meet the requirements for walls and ceilings in a food facility and are easily punctured or torn. Thus, insulation, where used, should be installed so that it is not exposed and is sealed off from food processing and handling areas. The type of insulation material used must also be considered. Fiberglass batting insulation should be avoided as it attracts insects and rodents, and the fibers may become airborne causing a contamination hazard. Acceptable materials may include: Styrofoam panels, foam glass, and urethane.
Due to heavy day to day exposure to a variety of chemicals and food products, the floor in a food processing and handling facility is the most difficult surface to maintain. Floors should be smooth, impervious, non-absorbent, corrosion resistant, cleanable and in good repair. For safety considerations, floors should not be so smooth that they cause employees to slip and fall. In addition to being constructed and sealed adequately, the floor should be installed to provide adequate slope for drainage and preven¬tion of pooled water. The most recommended are sealed concrete, epoxy sealed concrete, quarry tile, and glazed tile. Each of these materials would provide an acceptable floor surface if properly installed and maintained in good repair. Once the integrity of any of these surfaces becomes compromised, they can harbor microorganisms, especially in wet areas. Unsealed concrete floors should be avoided as they are highly porous and break down with continued exposure to chemicals. Once cracks, crevices, spalls, or other damage occurs, a concrete floor is especially vulner¬able to harboring microorganisms. Quarry tile or glazed tile floors require additional maintenance as grout lines can erode causing a multitude of problems. As mentioned for walls and ceilings, metal and wood must be avoided as a floor construction material.
Integrating the floor juncture, with the walls, must be actively addressed, to provide a smooth transition. PortaFab can provide several options and coving profiles, to provide this transition.
Floor drains are a major source of microbial contamination in a food processing facility. Thus, they require special attention. Floor drains should be of adequate number and size, appropriately located, designed and installed so that they are cleanable; and maintained in good repair. Circular, catch basket drains are most often recommended provided that they are appropriately sealed and grouted to the floor, and are maintained in good repair. Trench drains, although used in many operations, can have problems. A trench drain should be constructed and installed to provide adequate slope or grade ensuring there is no standing water in the trench. The grouting and sealing of trench drains at the floor junctures is also more difficult to maintain than the seals of circular drains.
Adequate lighting is important for all operations conducted in a food facility. This is especially true in cleaning and sanitizing and related operations. Recommended lighting levels vary between regulatory officials and other sources. Light fixtures should be of the type approved for food facili¬ties, and should be equipped with break resistant lenses or shatterproof shielding. The fixtures should be designed to be moisture resistant and cleanable.
Heating, ventilation, and air conditioning (HVAC) systems function to maintain the temperature and humidity of a facility. Day to day sanitary operations are dependent upon a properly functioning system for prevention of condensa¬tion as well as overall employee comfort. In addition, it is desirable to create positive air pressure differentials in critical or sensitive food handling rooms (e.g., packaging rooms). Because of these demands, the facility should have properly sized units and an adequate distribution system to do the job. Because HVAC systems have proven to be a source of contamination with pathogenic microorgan¬isms, certain sanitary construction, design, and installation features need to be considered. Systems should be constructed, designed, installed, cleaned, and maintained so that they are not a source of contamination. For example, the air supply should be located to not draw air from nearby sources of contamination (e.g., chemicals, bird droppings); adequate filters should be installed (and changed frequently); and duct work should be located outside of the processing areas. Finally, air handling systems should be designed to be cleanable. Interior surfaces should be sealed to provide for contamination control, and final air filtration is recommended.
Handwashing sinks, lavatories, or stations should be, conveniently located near food operations, of sufficient number based on the size and function of the operation, constructed and installed to meet plumbing codes includ¬ing appropriate backflow prevention and no submerged inlets, installed with faucets of sanitary design (preferably foot or electric eye operated), supplied with hot (not steam) and cold water in order to provide an adequate flow of water at 85° – 100° F, provided with an adequate supply of soap, single service towels, and a covered waste receptacle; and maintained, cleaned, and kept in good repair.
Employees need suitable facilities where they can safely store their clothes and other personal items. Maintenance and construction recommendations for walls, ceilings, and floors of locker rooms, restrooms, and related facilities should meet the same criteria as those for other areas of the facility. Lockers should be sealed to the wall and should have sloped, rather than flat, tops to prevent accumulation of dust and debris. Employee facilities should not open directly into processing or other critical areas (i.e. they should have vestibules). Most food regulations require a two-door separation between locker rooms or restrooms and food processing areas or food handling areas.
Permanent freezing and refrigeration rooms should meet the same sanitary construction and design criteria as other rooms in the food facility. With the exception of the refrigeration units and drain trays discussed below, an appropriately constructed and designed freezing or refrigeration room should present minimal sanitation problems. However, when modular type units are installed in a facility, they can be a source of dust and debris ac¬cumulation and contamination concerns. These units must be installed with sufficient space between the unit and the wall (approximately 18 inches) to allow accessibility for cleaning. Because the units or boxes are of flat-top design they tend to collect dust and debris, unless they are caulked and sealed to the ceiling. If they are free standing units, sufficient clearance should also be allowed above the unit to provide access for cleaning. Refrigeration units, due to coils, fins, and other dust collec¬tion points, can also be a source of contamination. These units should be designed and installed for adequate clean¬ing. A major contamination problem area is the condenser draining system. Drains, trays, and pans should be installed to prevent overhead contamination of stored food products, and should be flushed and cleaned daily. Drain lines exiting refrigeration rooms or boxes should be installed to drain into a floor drain, with an appropriate air gap, and should not drain directly into critical food processing and handling areas.
In addition to having sanitary walls, ceilings, and floors, a sanitary food processing and handling facility must also have designed-in (integrated) features to protect the food products from contamination. Facilities should be periodi¬cally inspected and evaluated for potential contamination of product due to the facilities themselves. Utility and water supply lines, and other accoutrements hung or attached to the wall or ceiling must be appropriately caulked and sealed to the wall or mounted in such a way to allow cleaning behind and around. Exposed threads should be minimized on hangers used for piping and other equipment or other attachments, as they ac¬cumulate dirt and dust. If threads are used, they should be of sanitary design to be cleanable. Wherever appropri¬ate, shielding should be provided over product conveyances and areas where food products are exposed. However, such shielding should be constructed of appropriate, cleanable material. Rather than horizontal or flat, the shielding should be gabled or sloped at a minimum of a 20 degree angle to prevent ledges that can collect dust and dirt.
Ideally, a facility should be designed to provide a flow pat¬tern for food products (as well as personnel and equipment) to prevent potential contact of the finished product with raw materials. Flow should be in one direction and follow a logical sequence from raw material handling to finished product storage as shown below.
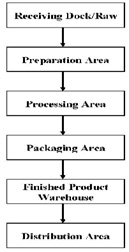
As much as is practicable, there should be a physical separation between raw and finished products and minimal entry into critical areas. Such physical separation should be accomplished by installation of walls and doorways with anti-back tracking features, and by adjusting air handling systems to provide positive pressure in finished product rooms. As the best physical separation can be undermined by human error or improper personnel flow, there should be an operational and philosophic separation between raw and finished product. This can be accomplished by barring employees working with raw materials from entering finished product rooms, this includes maintenance and janitorial staff. It is recommended that standard operating procedures be developed and implemented regarding product flow. In addition to providing procedures for personnel and equipment within the facility, the movement of equipment in and out of the facility by maintenance crews should also be considered. Color-coding is often used, with different colors identifying different areas of the facility. Color-coding can be applied to clothing (e.g., uniforms, frocks), cleaning supplies (e.g., brushes, brooms, pails), containers (e.g. ,pails, lugs), gaskets, forklifts, and any other equipment. Separation can also be accomplished by the installation of sanitizer systems (e.g., foot baths, spray systems).
inside entrance doors to critical areas. Visitors, suppliers, laboratory personnel, truck drivers, inspectors, management, and all other individuals should be made aware of operating procedures with regard to separation between raw and finished products. Self-inspections by quality assurance personnel, regulatory inspections, or tours should be done in a counter product traffic direction starting with finished product rooms and ending in raw material handling areas.
The FDA website has several Guidance for Industry publications that can assist in the design development of a properly constructed GMP Food Processing Facility.
